Corporate Building
147 / F , 8th Main, 3 rd Block,
Koramangala, Bangalore
560034, INDIA
Our corporate building (30,000 sq. ft) has the following functional teams:
- Clinical Operations
- Bioanalytical
- Quality Assurance
- GLP & Validation
- Information Technology
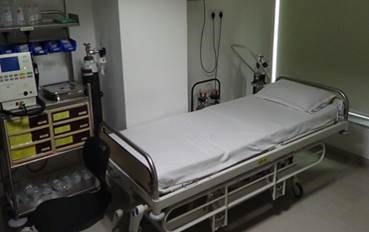
Clinical facility
147/F, 8th Main, 3rd Block,
Koramangala, Bangalore
560034, INDIA
- State-of-the-art facility
- 72 bed
- Screening Area
- Subject Housing Area
- Blood Draw & Sample Processing Are,
- Dining / Recreation Area
- 2 bed ICU
- Sample Storage Area
- Dedicated Pharmacy
BIOANALYTICAL
Our highly qualified and experienced staff develop methods that meet the high standards of FDA and ICH GLP. NCS’s quality driven processes and systems provide for reliable, reproducible and accurate data. NCS has experience with a variety of molecules in different matrices.
Our bioanalytical facility is approximately 10,000 sq. ft.
- Qualified, trained & experienced team of bioanalytical professionals
- Method development/validation for proprietary (NCE) and non-proprietary assays
- Bioanalysis of drug and metabolites in a variety of biological matrices
- Ability to develop and validate highly sensitive assays in low pg/ml range
- Ability to accommodate projects with rapid turn-around times
PHARMACOVIGILANCE
Norwich Clinical Services’ (NCS') pharmacovigilance, product surveillance and drug safety support services cover the entire product life cycle from development, through pre and post-marketing stages. The NCS team is your reliable partner in reaching compliance and maximal effectiveness of your pharmacovigilance/surveillance system and covering the complex regulatory requirements.
Our Pharmacovigilance/surveillance activities are in compliance with the FDA and the latest EU Guidance.
- Three successful FDA pharmacovigilance inspections (remote from client’s facility)
- Compliant XEVMPD software
- Electronic gateway established with EMA, all EU countries and with the FDA
- Vast experience in data migration
- Expertise in signal detection for more than 800 molecules
- Data mining of global safety databases including FDA
- Literature and regulatory site searches